Should drugs, natural compounds, food additives, pesticides and other compounds be tested for effects on the human gut #microbiome ? We now have the in vitro technology, called RapidAIM, to do the testing.
Results
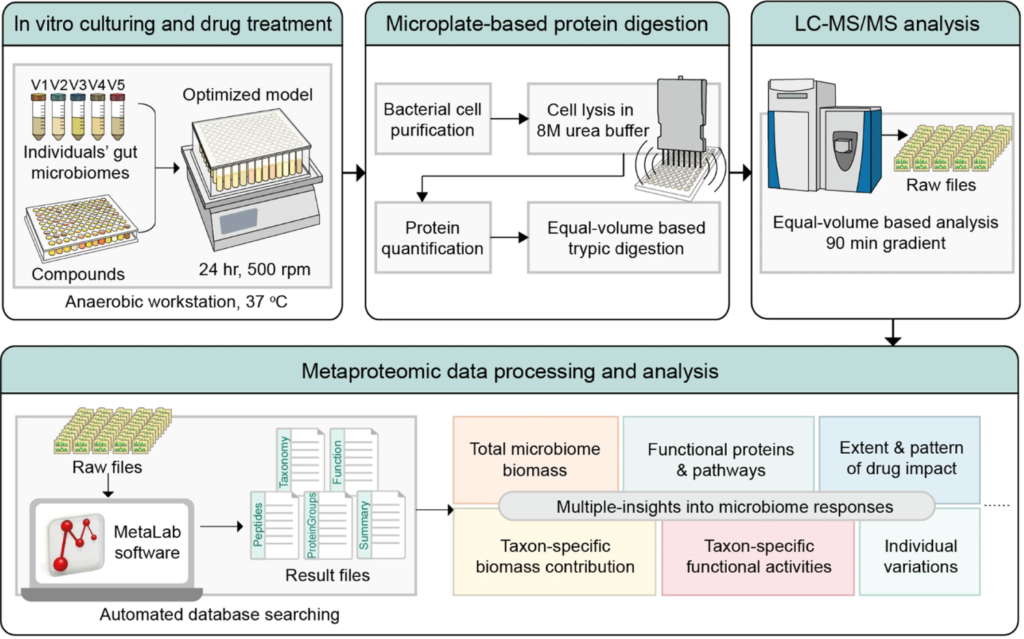
Here, we developed an approach to screen compounds against individual microbiomes in vitro, using metaproteomics to both measure absolute bacterial abundances and to functionally profile the microbiome. Our approach was evaluated by testing 43 compounds (including 4 antibiotics) against 5 individual microbiomes. The method generated technically highly reproducible readouts, including changes of overall microbiome abundance, microbiome composition, and functional pathways. Results show that besides the antibiotics, the compounds berberine and ibuprofen inhibited the accumulation of biomass during in vitro growth of the microbiota. By comparing genus and species level-biomass contributions, selective antibacterial-like activities were found with 35 of the 39 non-antibiotic compounds. Seven of the compounds led to a global alteration of the metaproteome, with apparent compound-specific patterns of functional responses. The taxonomic distributions of altered proteins varied among drugs, i.e., different drugs affect functions of different members of the microbiome. We also showed that bacterial function can shift in response to drugs without a change in the abundance of the bacteria.
Conclusions
Current drug-microbiome interaction studies largely focus on relative microbiome composition and microbial drug metabolism. In contrast, our workflow enables multiple insights into microbiome absolute abundance and functional responses to drugs. The workflow is robust, reproducible, and quantitative and is scalable for personalized high-throughput drug screening applications.